Mechanistic Bioenergetics and Computational Biochemistry
Our goal is to elucidate the mechanistic principles of biological energy conversion. To this end, we develop integrative biophysical methods that allow us to unravel how the catalytic power of enzymes arises from their structure, conformational dynamics, and biological surroundings.
Life is powered by a membrane-bound protein machinery that captures chemical and light energy to sustain the energy metabolism of our cells. At a molecular level, these processes are catalyzed by highly efficient protein complexes that transport charge (protons, ions, and electrons) across large molecular distances at remarkable catalytic efficiencies. Yet, despite recent structural advances, the mechanistic principles of these processes remain poorly understood and much debated. Our research aims to decipher the molecular mechanisms by which bioenergetic enzyme complexes catalyze long-range charge transport to power the cellular energy catalysis, and how their dysfunction results in human diseases. To address these challenging questions, we develop integrative biophysical approaches that combine powerful multiscale simulations and data-driven approaches with biochemical and structural experiments. Our multidisciplinary approach allows us to probe the energetics, dynamics, and functional principles on a broad range of timescales and spatial resolutions, and to rationally test mechanistic hypotheses in both natural and synthetic model systems.
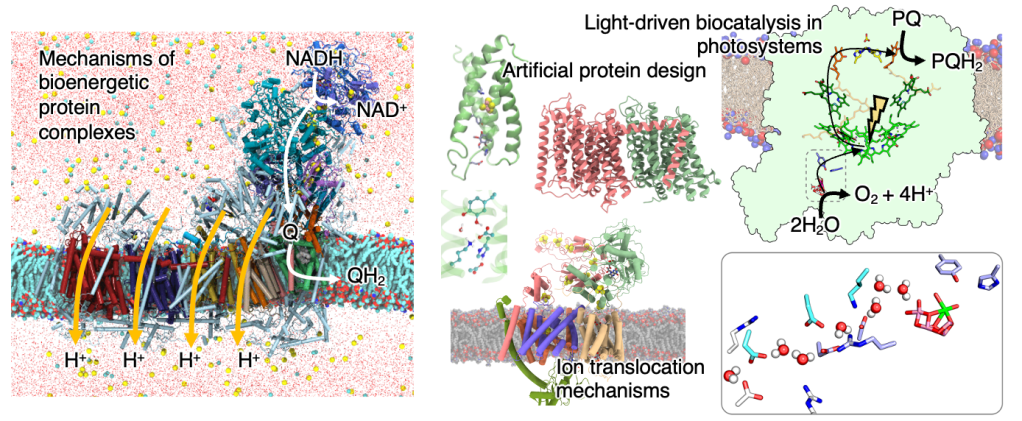
Our work aims to elucidate how biological systems capture and transduce energy. Of particular interest are the respiratory and photosynthetic protein machineries that provide unique systems to study the functional principles and intricate catalytic reaction mechanisms. The goal of our work is to decipher the exact time-ordered molecular principles of these fascinating proteins, and to provide a detailed physical understanding of how the biological function emerges from the protein structure. In this regard, we address: 1) how intricate bioenergetic protein complexes catalyze long-range charge transfer reactions near optimal thermodynamic efficiency across all domains of life, 2) general catalytic principles of redox-driven enzymes and synthetic models thereof, 3) how light-driven charge transport in photosynthesis enables water-splitting at various photon energies, and 4) develop multiscale computational methodology for the quantitative treatment of biochemical structure, dynamics, and reactivity. We aim to identify and probe key sites that modulate catalytic activities and long-range coupling effects in biological systems and systematically engineer new enzymes based on these; build up artificial energy transduction chains; and develop new integrative techniques that allow us to address the function of complex biological systems by combination of detailed multiscale simulations data, with mutagenesis experiments, spectroscopic proton pumping assays, and structural studies.

Our work focuses on understanding general molecular mechanisms of ion translocation across membranes, and long-range charge transfer as well as proton-coupled electron transfer (PCET) reactions in membrane-bound proteins central for the cellular energy metabolism. To establish a mechanistic understanding of these processes, we probe the functional principles of respiratory and photosynthetic enzymes, with a special emphasis on the Complex I superfamily, the protein complexes catalyzing oxidative phosphorylation and their supercomplex assemblies, while our work on Photosystem II and microbial ion-pumps give insight into light-driven charge transfer mechanisms. We study both modern and ancient redox and metalloenzymes, as well as in other intricate biochemical systems, such as molecular chaperones, which also employ long-range allosteric coupling mechanisms. To derive a general understanding of charge transfer principles in biology, we develop machine-learning based protein prediction methods together with our integrative methodology, with aims to rationally design redox-driven ion pumps. To this end, we aim to decipher how intrinsic electric field effects control biocatalysis and conformational dynamics of proteins, and how molecular locks and gates make these processes highly efficient and minimize energy dissipation effects
To address molecular reaction mechanisms of these complex systems, we develop computational multi-scale methodology that ranges from quantum chemical and hybrid quantum/classical (QM/MM) methods to large-scale classical and coarse-grained molecular dynamics (MD) simulations, which are combined with free-energy methods to study rare events. This allow us to address the structure, energetics, and dynamics of the complex chemical systems on fs-ms timescales for system up to millions of particles. Our multiscale computational methodology provide complementary information to many experimental techniques, and therefore offer powerful tools for probing mechanistic hypotheses at a molecular level. To obtain complementary information to our computational work, we also develop and perform biochemical, structural, and biophysical experiments that allows us to test, refine and validate our mechanistic models.
Selected publications
- Kim H et al. (2023). Quinone Catalysis Modulates Proton Transfer Reactions in the Membrane–Domain of Respiratory Complex I. JACS 145: 17075-17086.
- Saura P et al. (2022). Electric Fields Control Water-Gated Proton Transfer in Cytochrome c Oxidase. PNAS 119(38): e2207761119.
- Allgöwer F et al. (2022).Molecular Principles of Redox-Coupled Protonation Dynamics in Photosystem II. JACS 144: 7171-7180.
- Röpke M et al. (2021) Deactivation blocks proton pathways in the mitochondrial complex I. PNAS 118: e2019498118.
- Baumgart M et al. (2021) Design of buried ion-pairs in artificial proteins. Nature Comms 12: 1895, 1-10.
- Kaila VRI (2021)Architecture of Bacterial Respiratory Chains. Nature Rev Microbiol 19: 319-330.
- Bridges HR et al. (2020) Structure of inhibitor-bound mammalian complex I, Nature Comms, 5261: 11, 1-11.
- Schuller JM et al. (2020) Redox-Coupled Proton Pumping Drives Carbon Concentration in the Photosynthetic Complex I, Nature Comms 11: 494, 1-7.
- Mühlbauer ME et al. (2020) Water-gated proton transfer dynamics in respiratory complex I. JACS 142, 13718-13728.
- Mader SL et al.(2020)Conformational dynamics modulate the catalytic activity of the molecular chaperone Hsp90. Nature Comms 11: 11410, 1-12.
